Highlight
| Intro | A new efficient oxide coating technology that can be applied in less than five minutes could lead to dramatic improvements in the lifetime and performance of fuel cells. The fundamental principle behind this approach is maximizing the oxygen reduction reaction site of a platinum thin-film electrode, preventing the electrodes from aggregating at high temperatures. |
|---|---|
| Principal Investigator | Prof. WooChul Jung |
| Date | 2018-07-18 |
A new efficient oxide coating technology that can be applied in less than five minutes could lead to dramatic improvements in the lifetime and performance of fuel cells. The fundamental principle behind this approach is maximizing the oxygen reduction reaction site of a platinum thin-film electrode, preventing the electrodes from aggregating at high temperatures.
Fuel cells have emerged as a clean electricity generation system that does not pollute the air. In particular, solid oxide fuel cells (SOFCs) are beginning to gain a great deal of attention due to their higher power generation efficiency compared to other fuel cells. It is also advantageous to use other power sources than expensive hydrogen fuel.
However, the high costs and insufficient lifetimes caused by high temperatures needed to operate the solid oxide fuel cells have remained significant challenges to commercialization.
Recently, attempts to lower the operating temperature (< 600°C) of these devices by introducing thin-film processes have drew attention of researchers, with the resulting products known as thin-film-based solid oxide fuel cells.
In order to create enhanced device performance at lower temperatures, the research team, led by Professor WooChul Jung in the Department of Materials Science and Engineering, applied and developed oxide coating technology to maximize the oxygen reduction reaction sites of a platinum thin-film electrode and to prevent platinum electrodes from thermal aggregating.
The team succeeded in over-coating a platinum electrode with a new coating material called praseodymium-doped ceria (Pr,Ce)O2-, which has high conductivity for both electrons and oxygen ions and excellent catalytic properties for oxygen reduction reactions. As a result, electrode resistance was reduced by more than 1000 times, creating the potential for these electrodes to be used in high-temperature electrochemical cells.
In addition, they proposed that the high performance of thin-film-based oxide fuel cells’ oxygen electrodes could be realized through the nano-structuring of (Pr,Ce)O2-δ without any platinum.
Professor Jung said, “The electrode coating technology used in this study is of great technical value because of the utilization of affordable and mass-produced electrochemical deposition.” He added, “In the future, this technology will be feasible for replacing platinum electrodes in thin-film-based oxide fuel cells, and we expect that the affordable prices of this fuel cell will eventually boost market competitiveness.”
This research was described in Advanced Energy Materials in July and was featured as the Inside Front Cover and video abstract. It was supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) and the Korea Electric Power Corporation (KEPCO) Research Institute.
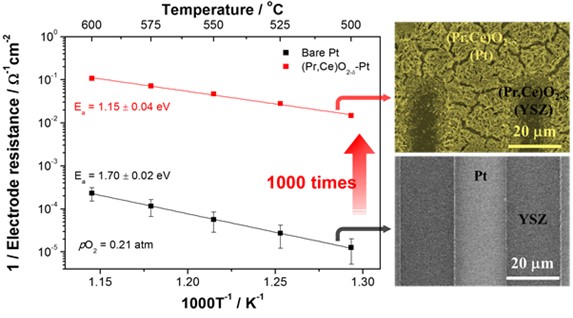
Figure 1. The change of electrode activity with and without overcoated (Pr,Ce)O2-δ nanostructures.






