Highlight
| Intro | Professor Do Kyung Kim from the KAIST Department of Materials Science and Engineering and his team succeeded in developing high-areal-capacity lithium sulfur batteries (Li-S batteries) by capturing polysulfide with carbon nanofibers. This research will provide new batteries to replace existing lithium rechargeable batteries, shifting the commercialization of related technologies ahead. |
|---|---|
| Principal Investigator | Prof. Do Kyung Kim |
| Date | 2018-05-14 |
Professor Do Kyung Kim from the KAIST Department of Materials Science and Engineering and his team succeeded in developing high-areal-capacity lithium sulfur batteries (Li-S batteries) by capturing polysulfide with carbon nanofibers. This research will provide new batteries to replace existing lithium rechargeable batteries, shifting the commercialization of related technologies ahead.
Electrical vehicles and large-scale energy storage systems necessitate the development of batteries with high energy density and cost effectiveness, and Li-S batteries are known to be one of the promising alternatives to the predominant lithium ion batteries.
With six times as much energy density, Li-S batteries theoretically thrust electric vehicle to twice the distance of lithium ion batteries. Therefore, they have been spotlighted as next-generation lithium rechargeable batteries because they can go up to 400km once charged.
However, several issues make it challenging to readily commercialize Li-S batteries. The low electrical conductivity of sulfur, volumetric expansion and contraction of the battery during charge and discharge, and permanent damage of the electrode caused by the dissolution of the lithium polysulfide into the electrolyte – known as the “shuttle effect” – are three of the biggest obstacles to commercial-grade Li-S batteries.
While there have been numerous attempts to curb, avoid, or alleviate these issues — such as the physical encapsulation of sulfur using various metal oxides or carbonaceous matrices — most of them entail utilizing zero-dimensional (0D) carbon materials. This encapsulation method has been somewhat effective in enhancing the electrical conductivity of sulfur while simultaneously tolerating some volumetric alterations and suppressing the shuttle effect. The downside of 0D carbon material-based encapsulation methods is their complicated synthetic processing and the limited mass loading of sulfur.
With this in mind, the team set out to employ one-dimensional (1D) carbon materials instead. Unlike the 0D case, 1D carbon materials render a large surface area and a long-range conduction path for electrons and lithium ions. Being 1D also solves the undesirable high-contact resistance problem frequently encountered by 0D carbon material-based encapsulation.
The key to developing the proposed material was to exploit the capillary force to decrease the energy associated with the dissolution of polysulfides. As such, carbon nanofibers (CNFs) were found to be suitable for high-areal-capacity lithium-sulfur batteries since capillary force acting between CNFs can take advantage of the high electrical conductivity with the suppressed dissolution of sulfides.
The research findings show that sulfur was successfully contained in between the CNFs by wetting due to the capillary force without the need for complicated synthetic processing, as in the 0D case. The research results indicate that the sulfur contained per unit area (mg/cm2) is five times greater for the newly implemented method, which then enabled the lithium-sulfur battery to achieve an areal capacity of 7 mAh/cm2, which amounts to as much as at most seven times that of conventional lithium ion batteries.
First author Jong Hyuk Yun stated that the unprecedented methods utilized in this study will help further and widen the progress of lithium batteries in general. Meanwhile, Professor Kim said, “This study brought us closer to commercial-grade high-capacity Li-S batteries, which are applicable for a wide variety of products, including electric vehicles, unmanned aerial vehicles (UAVs), and drones.”
This research, led by PhD candidate Yun, was published in the 18th issue of this year’s Nano Letters.
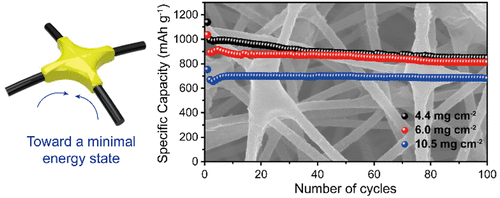
Figure 1. Electrochemical reaction leading to the containment of the sulfur within the carbon nanofiber and the corresponding specific capacity of the battery over a number of charge-discharge cycles

Figure 2. SEM images of the first discharged electrode containing lithium sulfide at the junction between the nanofibers, and the first charged electrode
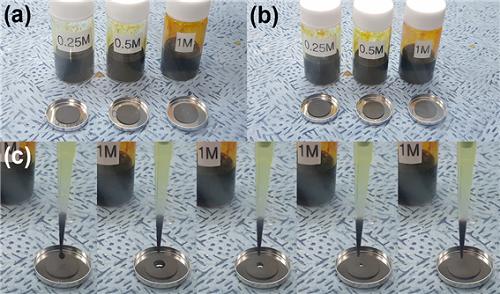
Figure 3. carbon nanofiber effectively absorbing liquid based lithium polysulfide






